
Warm Tip: If you want to know more details about equipment, solutions, etc, please click the button below for free consultation, or leave your requirements!
Gold vat leaching process is currently widely used in small-scale gold mines, because of a series of advantages including high recovery rate, strong adaptability to ore, low cost and simple process. The basic principle is first to leach the gold from the ore with cyanide solution containing oxygen, and then to extract the gold from the leaching solution with zinc powder or zinc wire.
The process steps of gold vat leaching and gold heap leaching are similar. The factors affecting gold heap leaching process have been discussed before. Now, we’re going to talk about the factors affecting gold vat leaching process.
The main cyanides that can be used for gold vat leaching process include: KCN、NaCN、NH4CN and Ca(CN)2. In the selection of cyanide, considering the solubility of cyanide to gold (The following table), chemical stability, consumption costs and other factors, most mines use sodium cyanide. However, because sodium cyanide will cause serious pollution to the environment, more and more mine owners choose cyanide replacement(low-toxic gold dressing agent) to extract gold, without changing the original cyanide process and equipment.
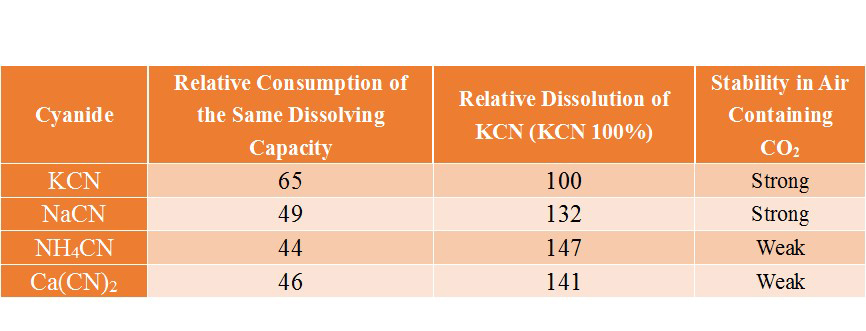
(The solubility of cyanide to gold)
2. Cyanide Solution Concentration
The concentration of the cyanide solution is the main factor that determines the rate of gold dissolution during gold vat leaching process, which has a greater impact on the vat leaching cycle and production costs. Excessive sodium cyanide concentration will cause waste of reagents, increase the cost, and cause great pollution to the environment. If the concentration of the cyanide solution is too low, the leaching speed will be slow and the leaching rate will be reduced.
The required concentration of sodium cyanide in the leaching solution is mainly related to the grade of the ore, and the required concentration increases with the increase of the Au grade. At the same time, different ores will contain different amounts of cyanide-consuming impurities. Generally, the concentration of the cyanide solution is between 0.03%-0.1%. The following table shows the conventional configuration of cyanide solution concentration.
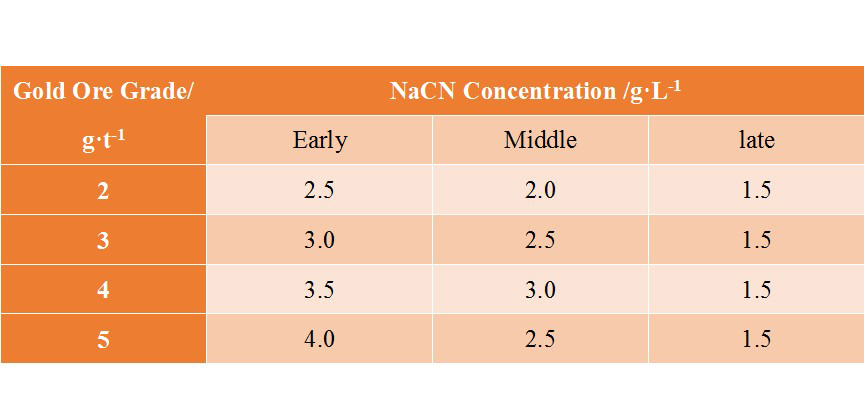
(The conventional configuration of cyanide solution concentration)
Ensuring sufficient alkalinity of the leaching solution is a necessary condition for gold vat leaching. If the pH value is too low or too high, the dissolution rate of gold will be reduced.
If the pH value is too low, NaCN will be hydrolyzed: CN-+H+→HCN↑. Part of the HCN generated will volatilize, causing the loss of cyanide agents. And because HCN is highly toxic, it can cause harm to humans and the environment. If the pH value is too high, some minerals in the gold ore will be decomposed, which will have an adverse effect on the dissolution of gold.
Therefore, too high or too low pH is detrimental to the leaching of gold. In the production process, the pH of the leaching solution should be strictly controlled, and the generally suitable pH is 10-11.
Gold vat leaching process has certain requirements on the temperature. The figure below shows the relationship between the dissolution rate of gold in sodium cyanide solution and temperature. Generally, the temperature at 15-20℃ can meet the vat leaching requirements.
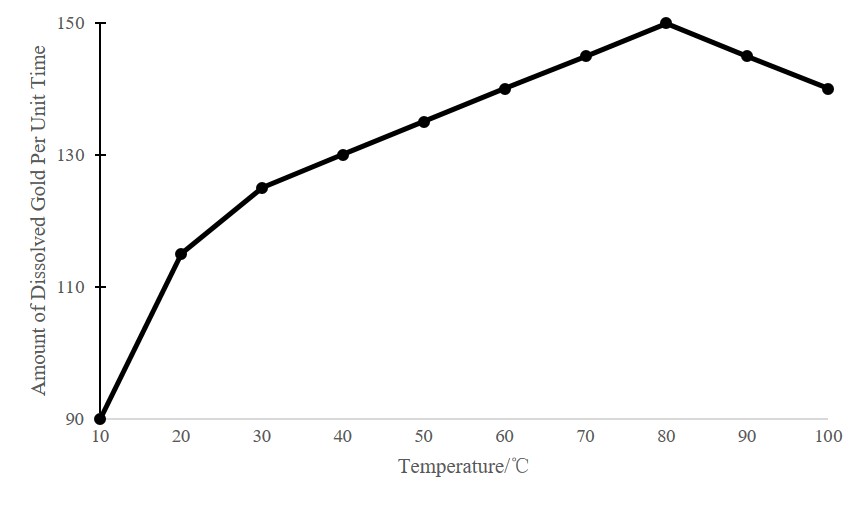
(The results measured under different temperature conditions)
The following table shows the results measured under different temperature conditions. It can be seen that when the temperature is as low as close to 5 ℃, production should be stopped.
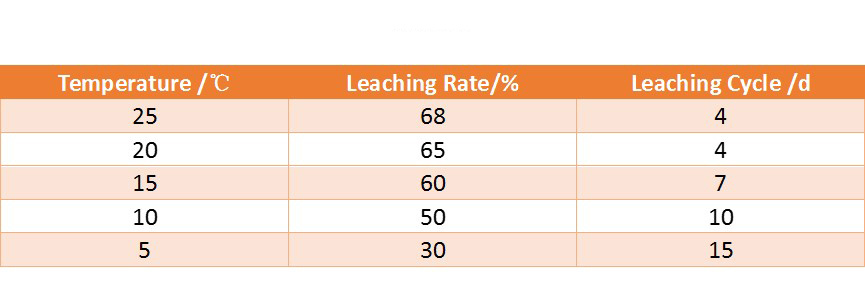
(The results measured under different temperature conditions)
For some minerals containing non-precious metals, pretreatment is needed to reduce cyanide consumption and improve leaching rate. Common methods include roasting, pickling, etc. You can choose the appropriate method according to the type of mineral. Gold will not change during the roasting process.
The size and shape of the gold particles exposed to the ore will affect the gold leaching rate. The larger the gold particle size, the slower its dissolution rate. Therefore, gold ore should be pre-ground before cyanidation, so that gold particles are fully exposed, which will improve the vat leaching rate and shorten the vat leaching cycle.
Slurry concentration and slime content directly affect the diffusion rate of reagent and the react between reagent and gold particles, so it is necessary to reduce slurry concentration for ore with much slime.
Proper agitation can accelerate the dissolution of gold, shorten the vat leaching cycle, and improve the vat leaching rate.
Cyanide selection, cyanide solution concentration, leaching solution pH and temperature will all affect the efficiency of gold vat leaching process. Although the gold vat leaching process is widely used at present, due to the lack of technical strength in some small mines, resulting in high production costs, pollution, low gold leaching rate and recovery rate, which affect the economic benefits of gold processing plants. Therefore, in the actual production of gold vat leaching plants, you should consider these factors in advance to improve the gold leaching rate and recovery rate, shorten the leaching cycle, and achieve the best economic benefits.
14 Reasons and Solutions of Poor Gold Leaching Effect
 0
0
 4740
4740
2Which Kind of Gold Mining Process You Should Choose?
 2
2
 3931
3931
3The Common Gold Mining Process – Gravity Separation Method
 0
0
 4884
4884


What Are the Differences Between CIP and CIL?
 10407
10407
 0
0